- Immune-oncology: Our pre-clinical research and development efforts focus on pioneering testing methods to validate therapeutic targets along the immuno-oncology axis, enhancing your novel therapeutics’ potential to harness the immune system’s ability to prevent, identify, control or kill tumour cells. We leverage cutting-edge technologies and methodologies to uncover and better understand the mechanisms behind these abnormalities on a molecular level, elucidating the true nature of your drug to combat these conditions.

Immunology CRO
Pre-clinical
Nexus BioQuest offers a custom-tailored approach to providing CRO services specific to immunology pre-clinical trials. Our focused expertise enables us to offer a specialised and personalised perspective on the development and management of your drug development strategy, adhering to all regulatory standards. We strive to elevate the prospective outcomes for your drug development programme, ensuring that medicines in the early development phase yield preliminary efficacy, toxicity, pharmacokinetic understanding, and safety information.
Our dedicated team of expert scientists and consultants are fully equipped with professional hands-on experience in analysing, designing, and executing pre-clinical studies with utmost scrutiny. Our mission is centered around patient priority, which brings our focus to providing expertise and innovative solutions to accelerate your immuno- therapeutics to the clinic in a timely manner.
Our Therapeutic Areas of Focus
Our focused expertise in immunological research allows us to deep-dive into various aspects of the field, ensuring comprehensive support for your drug development programmes.
Autoimmunity
Our team of specialised immunologists is committed to advancing treatments that address some of the most challenging and critical conditions in immunology.
- Autoimmunity: Autoimmune diseases such as rheumatoid arthritis and multiple sclerosis are suffered by many. Here at Nexus BioQuest, we aim to address the underlying causes of such conditions with a meticulous and stringent approach, ensuring that your novel therapeutics’ mode-of-action is fully understood. Our current drive to test more targeted therapeutics stems from our goal to potentially modulate or reprogramme the immune system’s framework with your drug. This way, we are bringing preliminary promises to patients who require this type of treatment into the clinic.
Inflammation
- Inflammation and Neuro-inflammation: The study of inflammation is a continuous focus for many scientists, aiming to address the issue of uncontrolled inflammation that can lead to conditions such as heart disease, Crohn’s disease, and Huntington’s disease. Here at Nexus BioQuest, we are experts in modelling inflammatory processes in vitro, offering specialised testing services for your novel immuno-modulators to hit the market.

Drug Discovery Tool
Find the right immune assay for your therapeutic area, modality and target using our interactive Drug Discovery Tool.
Why Partner with Nexus Bioquest?
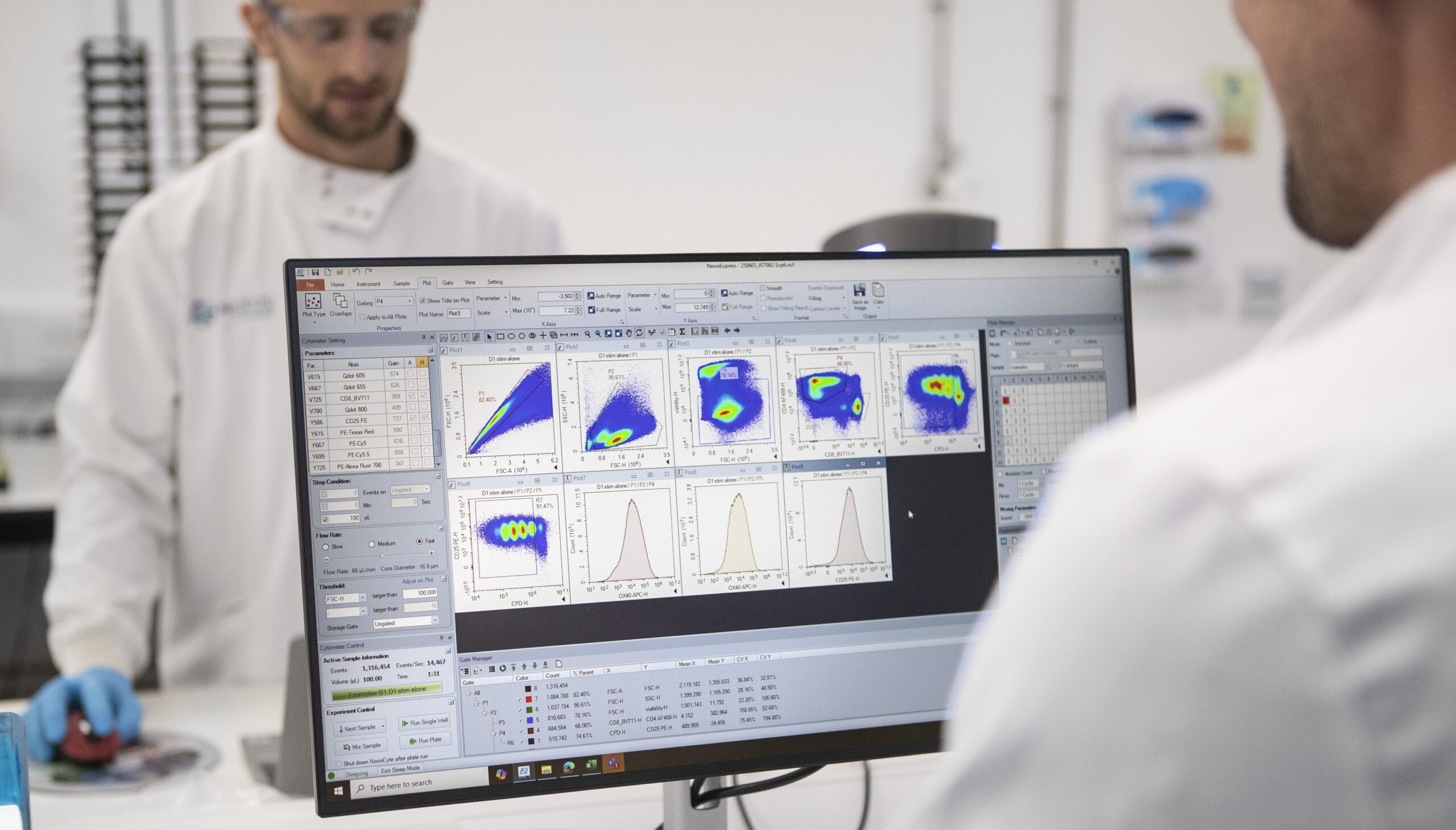
State-of-The-Art Technology Platforms
Using state-of-the-art technology, our team of expert immunologists can execute custom pre-clinical programmes that ensure your drug’s safety profile. These are some of the technology platforms we utilise here at Nexus BioQuest to conduct studies that make a real difference.
- ImageXpress Confocal HT.ai High-Content Imaging System
- Cytena CellCyte Real Time Imaging
- Novocyte Pentium Flow Cytometer
- Olink Proteomics
Bespoke Approach to CRO Services
Partnering with Nexus Bioquest is partnering with ongoing immunological innovation. We provide clients with immunological expertise following this CRO framework we believe that fosters ongoing collaboration through each phase:
- Consultation – Engagement with our specialised team of immunologists with a broad expertise in planning, operating and assessing pre-clinical studies.
- Study design and pricing – Collaborative and tailored approach to a drug discovery programme, with a detailed breakdown of pricing for each service.
- Contract and proposal formulation – Comprehensive and professional documentation of trial design, ensuring maximum clarity on both ends.
- Laboratory execution – Implementation of pre-clinical in vitro studies supplemented with expert oversight over preliminary drug development programme.
- Data package and recommendations – Proficient and efficient communication of harvested data, with clear and precise data-driven feedback for the next steps of your drug development programme.

Our Principal Immunologists

Sally Woodman

Christopher Rice

Silvia Rosini

Helen Tunbridge
What our clients say

Talk to a Scientist
Use our instant calendar booking service and speak to a Scientist at your convenience. With no delay, a call gives you immediate answers to your research questions.





