Monocyte
Inflammation Assays
- Inflammasome/LPS cytokine release
- Predictive immunotox CRA
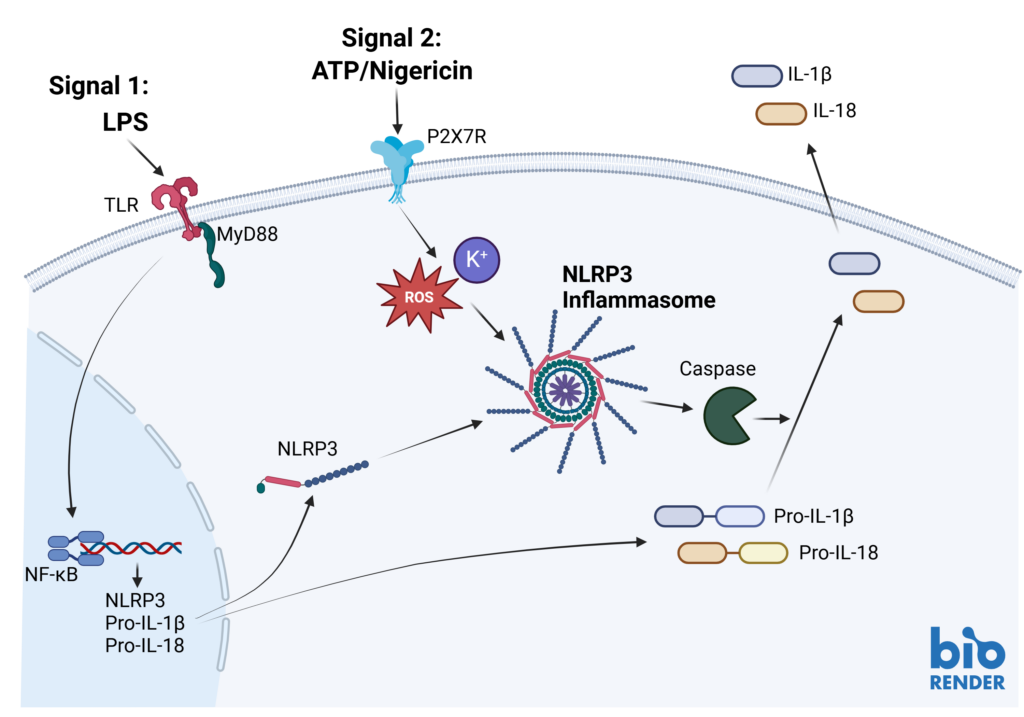
Inflammasomes are key signaling platforms within the immune system. Inflammasome complexes form in response to infection, tissue damage or metabolic imbalances. Once formed the inflammasomes activate Caspase 1 which in turn activated the pro-inflammatory cytokines IL-1β and IL-18. Targeting the inflammasome and the resulting signaling pathways is a useful tool in modulating the immune system.

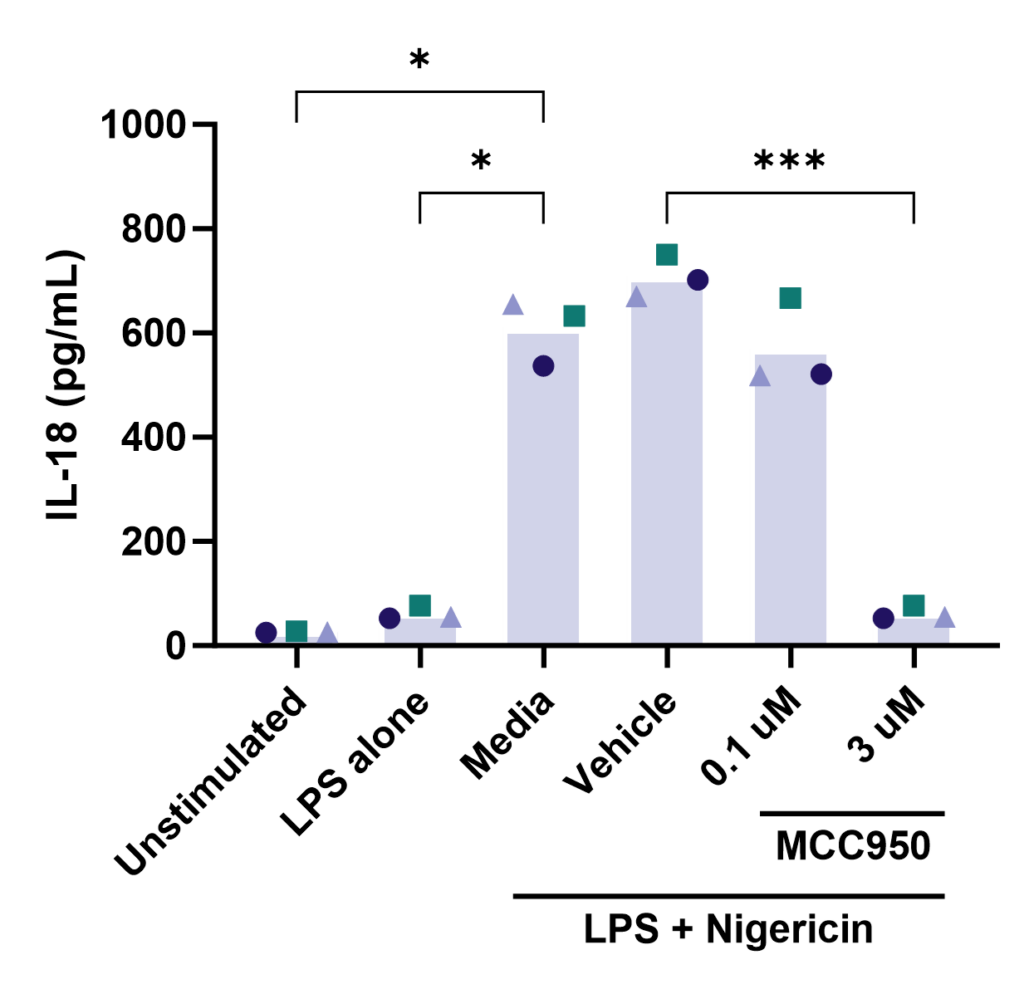
Effect of MCC950 on Inflammasome activation – IL-1β and IL-18.
PBMC from three healthy donors were pretreated with MCC950, vehicle (DMSO) or media for 1 hours prior to stimulation with LPS for 4 hours. Nigericin was then added for a further 45 minutes prior to harvest of the cell culture supernatant for quantification of (A) IL-1β and (B) IL-18 by TR-FRET and ELISA respectively. Data points show mean +/- SEM of technical replicates. One-way ANOVA with Dunnett’s multiple comparisons test comparing stimulated (media) to unstimulated, LPS alone, vehicle and MCC950 to vehicle; *<0.05, **<0.01, ***<0.001.
Other formats: PBMC, macrophage subsets, microglia
Cytokine release assay can be used to “de-risk” any potential unwanted effects of novel biotherapeutics in accordance with FDA and EMA recommended guidelines.
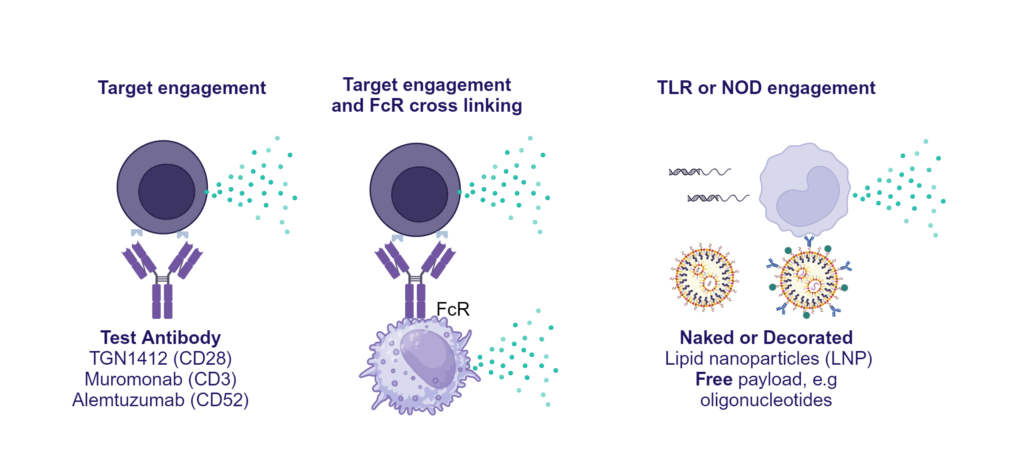
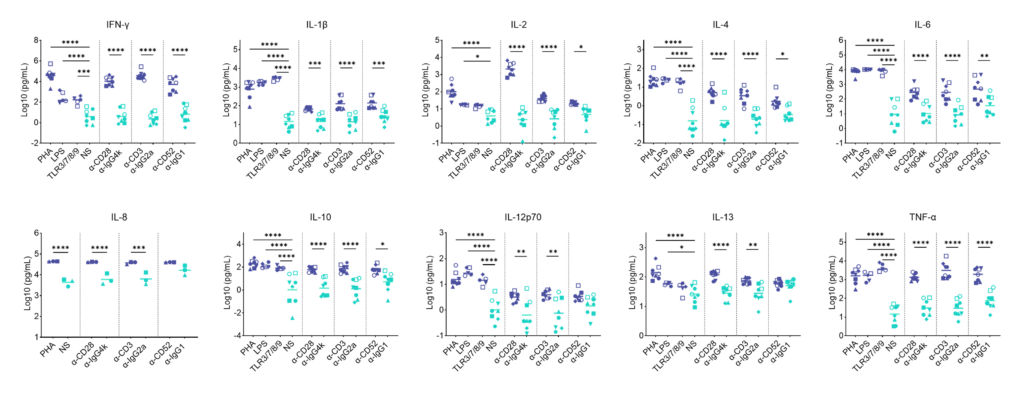
Preclinical safety PBMC cytokine release assay (CRA)
PBMC solid phase CRA: release of IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13 and TNF-α in response to a panel of antibodies coated onto plastic or TLR stimuli or polyclonal T cell stimuli (PHA).
PBMC isolated from buffy coats were added to wells coated with a-CD28 (TGN1412), a-CD3 (Muromonab), a-CD52 (Alemtuzumab) or with isotype control antibodies, or were stimulated with PHA, LPS, or a cocktail of TLR3/7/8/9 agonists. Cytokine release was measured at 48hr post stimulation. NS = Non-stimulated; Graphs show log10 transformed data with mean of 10 donors indicated. One way ANOVA with Sidak’s multiple comparisons test performed on log10 transformed data with significance indicted as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Note for IL-8 where values were above the ULOD these were assigned ULOD value.
Other readouts: frozen disease PBMC
Other formats: Whole Blood CRA
Other immuno-tox assays: include ADCC/ CDC/ ADCP
Preclinical safety whole blood cytokine release assay (CRA)
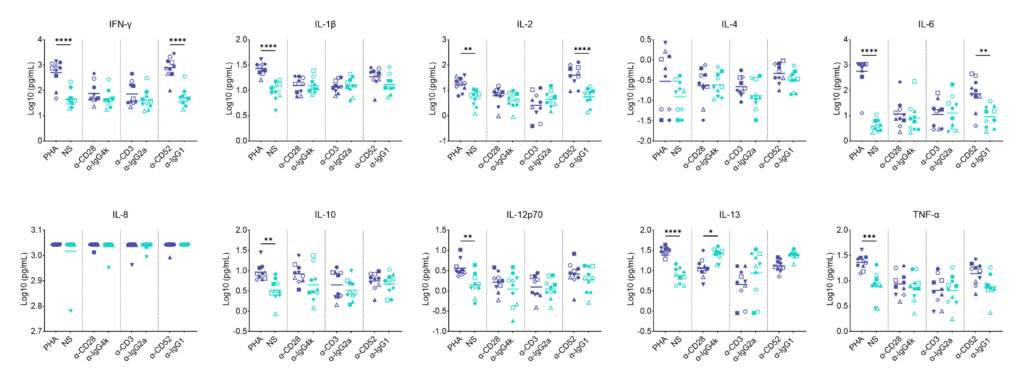
Whole blood soluble CRA; release of IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13 and TNF-α in response to a panel of antibodies or polyclonal T cell stimuli (PHA).
Whole blood was added to wells containing a-CD28 (TGN1412), a-CD3 (Muromonab), a-CD52 (Alemtuzumab) or with isotype control antibodies or were stimulated with PHA. Cytokine release was measured at 48hr post stimulation by MSD. NS = Not stimulated. Graphs show log10 transformed data with mean of 10 donors indicated. One way ANOVA with Sidak’s multiple comparisons test performed on log10 transformed data with significance indicted as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. Note for IL-8 where values were above the ULOD these were assigned the ULOD value.

Drug Discovery Tool
Find the right immune assay for your therapeutic area, modality and target using this interactive Drug Discovery Tool.
